Random Fun Facts - Mon, Jan 1, 0001
Just a collection of random fun facts :D
- Gloopy ethanol
- Supercooled water and strawberries
- I tried explaining ROP chains to an AI
- Pericyclic Boc Deprotection
- I went to the same restaurant 3 times without knowing
- Shiny Chemistry, All from Coffee
- Extracting DNA from Strawberries
Ethanol becomes gloopy at low temperatures
Did you know that ethanol has a freezing point of -114.1°C :O
If you cool ethanol down to a decently low temperature (say -50°C and lower), its viscosity will increase quite noticably, and it will turn from a nice flowy liquid to this strange thick gloopy substance. It does definitely make sense for the viscosity of a liquid to increase with decreasing temperature, but I guess we just don’t notice it that much with water as the freezing point of water is not very low.
Supercooled water and strawberries
You might have seen that party trick where a bottle of water almost instantly goes from liquid to solid with a little agitation. This is due to water being cooled to below its freezing point – also known as supercooling. Generally, the water used has to be very pure; this is as any solid impurities can act as nucleation sites for ice crystals to form. Technically, it is possible to supercool water all the way down to −48.3°C, where homogeneous nucleation then occurs.
Our university held a demonstration lecture to promote Chemistry to the public, and I was in charge of preparing the supercooled water. We had these annealed glass tubes (so that the edges would be free of scratches that could potentially act as nucleation sites) which we would fill with HPLC grade water. The tubes would then be cooled in an 1:1 ethanol-water mixture held between −7°C to −13°C, and the water temperature would be monitored via thermocouples. This was the setup that we used:

Maintaining the water in a supercooled state and getting the temperature as low as possible was actually way harder than expected! The water had to be incredibly pure, and all equipment had to be thoroughly rinsed and free of dust. And even so, I had to minimize any sort of agitation to the setup – the shockwaves from demo explosions happening in the lecture theatre have actually managed to set off my tubes as well! Basically, the entire setup was exceptionally finnicky.
While researching methods to reach a lower temperature, I happened to come across this paper about supercooling in frozen strawberries (apparently the texture and appearance of frozen strawberries is dependent on the growth of the ice crystals?? 0.0). According to the paper, if the strawberries were cooled to 2.9°C, held at that temperature for a period of time, and then cooled further, they would have the highest supercooling capacity. So I was like, hey, why not try this on my tubes? I would cool the tubes from room temperature to 2.9°C, hold them at that temperature for a couple of minutes, and then cool them down further. I am not sure whether this is due to confirmation bias, but I believe that the tubes of water were indeed able to reach a lower temperature! The lowest temperature that I was able to reach was −7.6°C.
During the demonstration, the lecturer would take one of the tubes, and touch the thermocouple probe to the side of the wall to initiate crystallization. This is how it looked like:

Nevertheless, I think that this was really interesting – if you do know whether “annealing” the tubes at 2.9°C would actually have improved their ability to reach lower temperatures, do definitely let me know!
I tried explaining ROP chains to an AI
I made an AI listen to me yap about ROP chains for fun and apparently the AI likes my ROP chain analogy.
Huh.
Damn, that's pretty complicated.
But I think I understand.
Yeah I guess so...
...
Now that I'm thinking about it though, that's a surprisingly decent analogy...
Boc protecting groups falling off at high temperatures may be due to a pericyclic reaction
If you have worked with Boc groups, you will notice that under high temperatures, they tend to fall off, even in the absence of an acid like TFA D:
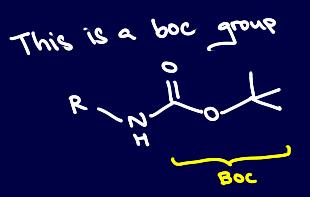
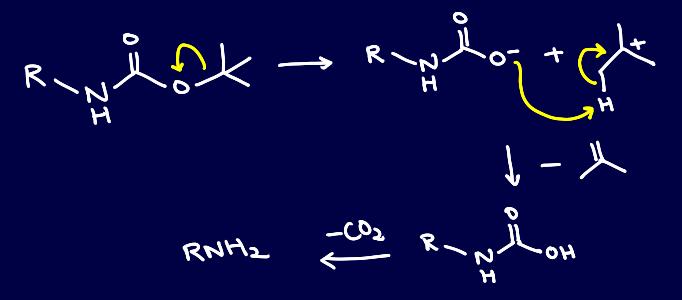
This is due to the thermal deprotection of Boc, which is actually regarded as a potentially greener pathway since no catalyst nor acid is required. The mechanism is as such:
However, one of my supervisors suggested that deprotection could potentially go via a pericyclic mechanism:
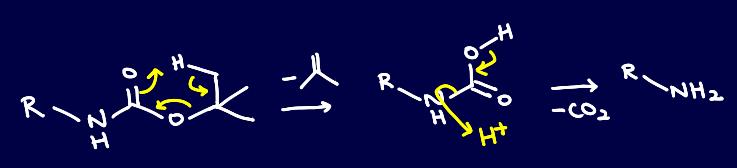
Pretty cool, eh?
Vinitus, Ciudad Condal and Cervecería Catalana are basically the same restaurant
So I took a trip to Barcelona this summer. I absolutely LOVE food, so I spent the entire trip stuffing my face with tapas (I LOVE TAPAS ❤️). A Google search recommended Vinitus, Ciudad Condal and Cervecería Catalana, so we planned to hit those three places. After going to Vinitus for lunch, we went to Ciudad Condal for dinner, and realized that the menu was basically the same 0.0
A quick search later on revealed that all three restaurants were run by the same group. My family originally intended to take Cervecería Catalana off the itinerary after finding out, but we’ve already been to two of the restaurants, so why not complete the tour? After much pestering, they finally gave in, and we went to three of basically the exact same restaurant in Barcelona :D (the food is excellent though. I highly recommend it, but if you wanna find out my absolute favourite tapas place so far, read on.)
…While writing this much later on, I realized, that there is a FOURTH restaurant – La Flauta (the name of the restaurant group itself). I thought that I have completed the go-to-the-exact-same-restaurant tour, but looks like I now have to make another trip to Barcelona to truly finish it :O
As of now, my absolute favourite tapas place is Güell Tapas. Sure, it’s in a touristy area, but the arroz negro and Andalusian chipirones are INCREDIBLE. We went there twice (I think you can see a pattern by now :D).
Shiny Chemistry, All from Coffee
I helped out with our department’s open day again this year, and for the demonstration lecture, we had a chemiluminescence demonstration! For those who don’t know what chemiluminescence is, it is basically the phenomenon where photons (light) are emitted as a result of a chemical reaction. A classic example of this is the oxidation of luminol, which is beautifully demonstrated by Thoisol2 here.
However, as the theme of the lecture was “Free Range Chemistry”, the professor giving the lecture wanted all the compounds used in the demonstrations to be derived from natural sources! This brings us to coffee – apparently, people have been very interested in the chemical composition of coffee! There are literally multiple papers where people brew coffee and then use fancy spectroscopic techniques to study the components of its aroma – check out “Aroma analysis of coffee brew by gas chromatography-olfactometry” for an example. Interestingly, the compounds vanillin (responsible for the smell and flavor of vanilla) and perylene (a polycyclic aromatic hydrocarbon) have been detected in coffee, which when put together, can result in chemiluminescence!
Jilani et. al. from Salisbury University wrote a paper “A Greener Chemiluminescence Demonstration”, where they synthesized divanillyl oxalate from vanillin and oxalyl chloride, and mixed it with perylene, ethyl acetate, sodium hydroxide and hydrogen peroxide to give a nice shiny blue color.
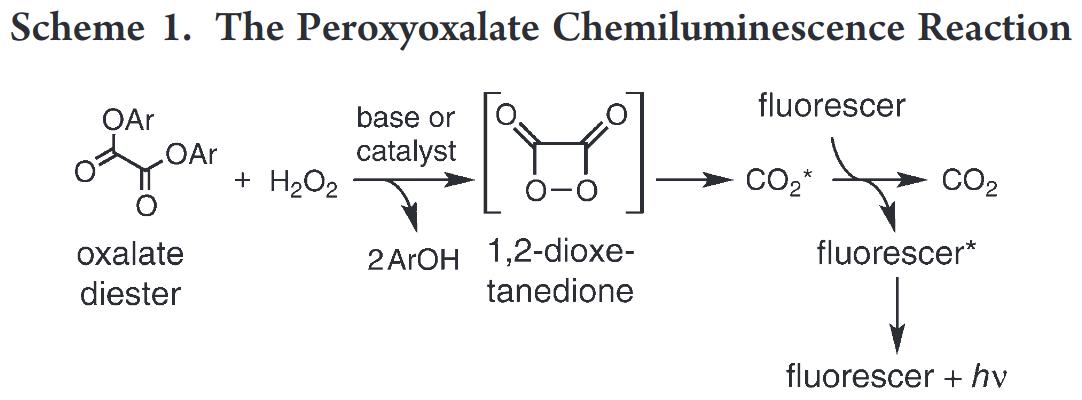
The way this works is that the oxalate diester will react with hydrogen peroxide in the presence of base to generate an unstable 1,2-dioxetanedione, which then decomposes into electronically excited carbon dioxide. The excited carbon dioxide then transfers energy to perylene, resulting in excited perylene. When excited perylene decays back to the ground state, it releases a photon, resulting in that nice, shiny blue light we see.
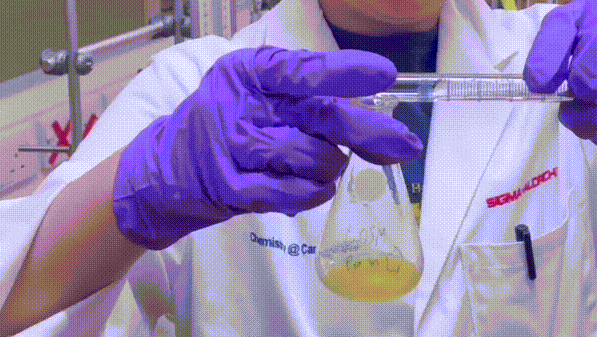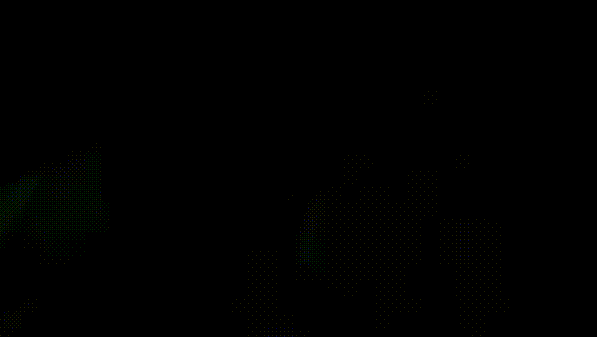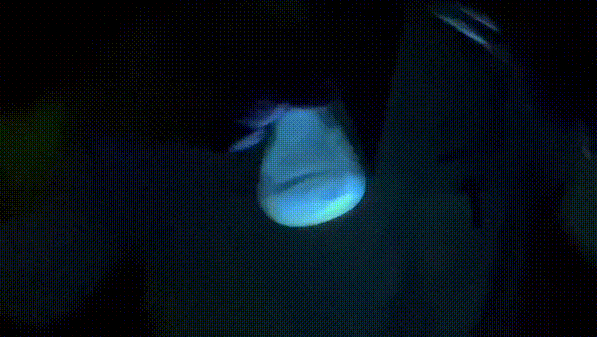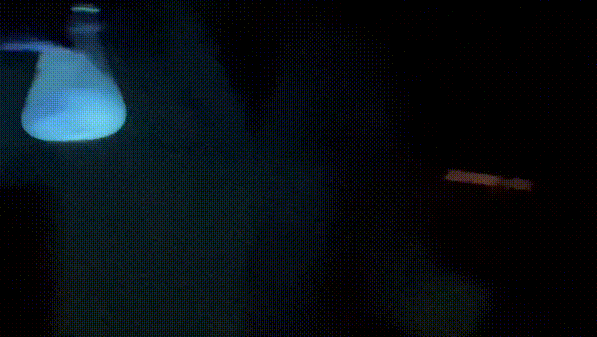
Shinyyyyyyyyyyyyy.
In the paper, the reaction is done at a 3 gram scale (with respect to vanillin), which is already a lot of material from a synthetic chemistry perspective. However, because each demonstration required around 4 grams of solid, and there were four lectures and one rehearsal, we needed a huge amount of material! So, we scaled up the reaction 10 times, which meant that each run, we were using almost 32 grams of vanillin… I ended up working with 2-3 liter conical flasks, and did my extractions in a 2 liter separating funnel – largest extraction I’ve ever done in my life! Check out this mess of a fumehood:

The rest of the lecture was also really fun. There was the “exploding rock” made from dry calcium oxide (as it reacted with water to form calcium hydroxide), exploding nitrogen triiodide, exploding nitroglycerin, gunpowder which caught fire, exploding yellow powder, nitrocellulose that burst into flames… You get the idea. Chemists, really, REALLY, R E A L L Y like fire. And blowing things up. (Last year I also got to blow up a giant hydrogen balloon. That was really cool! 💥💥)
Update: I had to clean up the fume hood. This is after the clean up:

Extracting DNA from Strawberries
…Sounds like a normal, fun little science demo for little kids, right? Hmmm…
So while I helped to synthesize the divanillyl oxalate for the demonstration lecture, as I was not directly involved in the lecture itself this time, I was apparently trusted to lead a group of undergrads to run the DNA extraction demonstration for Open Day (can I really be trusted to lead undergrads??? 🤔)
When I received the risk assessment from the person in charge, this was what was on it:

Hmmm…… We underestimate the creativity of children.
On the day itself, the team did up a little sign on the whiteboard, complete with reminders to well, Do Not Eat.

I would say that most of the kids were pretty well behaved, but there were those specific few that would Try To Eat. Even considering that the mixture had mashed up strawberry 🍓, pineapple juice 🍍 (pretty nice so far), salt 🧂 (doesn’t exactly go well with fruits…), dishwashing liquid 🧼 (probably gross), and absolute ethanol 🍷 (not kid friendly). A few of them tried to taste or drink the solution, one of them tried to drink the absolute ethanol. However, the absolute champion did something even better.
So here’s the thing: we were in a Chemistry teaching lab. And usually, in a Chemistry lab, the sinks would have multiple taps – one for deionized water, one normal tap, and a tap with a hose for washing stubborn glassware. Apparently, one of the kids decided to stick the hose into their mouth to try to drink the water 🤯!!! This resulted in a very loud “DON’T DO THAT!!!” from one of the demonstrators – it was incredibly funny. Another really funny thing that happened was that one parent stuck a “Do Not Eat” sticker on their kid.
Other pretty funny things they said included:
Kid: “If I ate strawberry DNA, will I turn into a strawberry? 🍓”
Kaligula: “No!!! Don’t eat it!!! But yes, when you eat normal strawberries, you are eating the DNA.”
Kid: “But if I eat it and turn into a strawberry, you can’t stop me! I will roll away!”
Overall it was extremely fun, and both the kids and their parents were actually very interested in the practical! Once all the visitors left, the team and I took the liberty to make the most disgusting strawberry DNA solution ever (note the disgusting beaker of crap in the background – would you really want to eat that???):

And finally, remember:
